检测到您当前使用浏览器版本过于老旧,会导致无法正常浏览网站;请您使用电脑里的其他浏览器如:360、QQ、搜狗浏览器的极速模式浏览,或者使用谷歌、火狐等浏览器。
 下载Firefox
下载Firefox
检测到您当前使用浏览器版本过于老旧,会导致无法正常浏览网站;请您使用电脑里的其他浏览器如:360、QQ、搜狗浏览器的极速模式浏览,或者使用谷歌、火狐等浏览器。
 下载Firefox
下载Firefox
js33333金沙线路检测定量生物学中心/化学与分子工程学院
学术报告
题 目: How does protein disorder function?
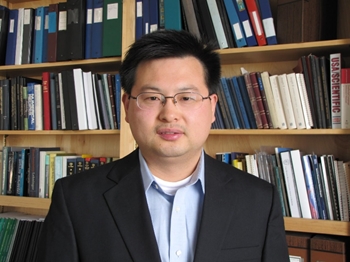
Associate Professor, School of Medicine, Case Western Reserve University
时 间: 7月18日(周二)13:00-14:00
主持人: 来鲁华 教授
摘 要:
How a sequence of amino acids determines the structure-function relationship remains in the “dark” for disordered proteins. To unravel the molecular mechanism of single-site phosphorylation in these disordered chains, we use a combination of in vitro protein phosphorylation, complementary biophysical approaches of small-angle X-ray scattering and nuclear magnetic resonance spectroscopy, and cellular gene expression, with a specific focus on the human estrogen receptor (ER). The phosphorylation at Ser118 that alters the ER transcriptional function results in the modulation of hydrophobic clustering of residual structures in its disordered region, a new and emerging phenomenon that cannot be explained by conventional electrostatic interactions associated with phosphorylation. Our findings provide a sequence-structure-function paradigm for a disordered protein by establishing a direct link between phosphorylation-induced conformational changes and the activation function of this disordered protein as a promising avenue to block ER transcriptional activation.
After completing his Ph.D. in Biophysics from UC San Diego in 2006 and postdoctoral training at the University of Chicago in 2010, Dr. Sichun Yang joined CWRU to initialize inter-disciplinary projects on the biophysics and drug discovery of Estrogen Receptor (ER), a key driver of breast cancer. Their work on integrative biophysics has enabled them to analyze highly flexible biomolecules such as ER proteins (e.g., inter-domain communication and intrinsic disorder). These achievements provide a solid groundwork for facilitating the development of new therapeutics.